The Science behind Tirzepatide
Amino Acid Sequence: YE-Aib-GTFTSDYSI-Aib-LDKIAQ (C20 fatty acid) AFVQWLIAGGPSSGAPPPS
Note: Aib is a non-coded (non-proteinogenic) amino acid – H2H-C(CH3)2-COOH
Molecular Formula: C225H348N48O68
Molecular Weight: 4813.527 g/mol
PubChem CID: 156588324
CAS Number: 2023788-19-2
Synonyms: P1206, LY3298176
Source: PubChem
Simply put, Tirzepatide increases the release of insulin from the pancreas resulting in improved glucose control. Research shows that, in individuals with Type 2 diabetes, Tirzepatide decreases hemoglobin A1c (HbA1c) levels by 2.4% after six months. The peptide also appears to aid in weight loss, showing a dose-dependent relationship and helping individuals lose as much as 11 kg (25 lbs) over six months[1], [2].
It isn’t just that Tirzepatide increases insulin release though. Research suggests that the peptide actually improves the function of pancreatic beta cells, the cells that make and release insulin. Studies suggest that Tirzepatide may actually make beta cells more effective at processing insulin, which leads not just to increases in insulin levels in the bloodstream, but decreased stress on the beta cells themselves. This may, in turn, help to slow the progressive nature of type 2 diabetes.
Research shows that Tirzepatide doesn’t just increase insulin levels at random though. It appears to do so only in response to increased blood glucose levels. During fasting, Tirzepatide actually decreases insulin levels and thus helps to increase insulin sensitivity over time. It also decreases fasting levels of glucagon, which are thought to exacerbate hyperglycemia by interfering with hepatic glucose metabolism. Overall, these changes are a big part of the reason Tirzepatide has a profound effect on glucose and, ultimately, HbA1c levels[3].
How Does Tirzepatide Work?
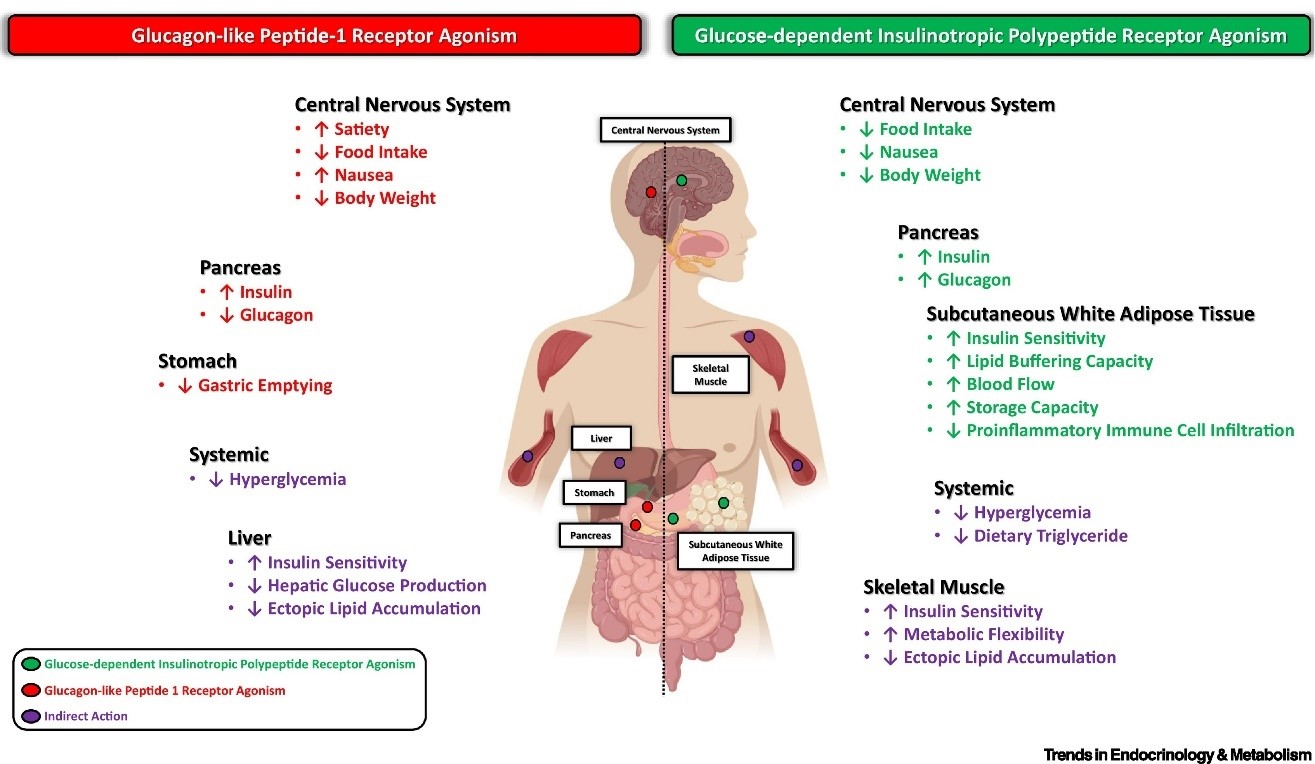
Tirzepatide is a dual agonist of the gastric inhibitory polypeptide receptor and the glucagon-like peptide-1 receptor. Action at these receptors appears to have synergistic effects that make Tirzepatide more effective than strict GLP-1 agonists that are already approved for the treatment of type 2 diabetes. The affinity of Tirzepatide for the GIP receptor is greater than its affinity for the GLP-1 receptor.
Gastric inhibitory polypeptide, which is also referred to as the glucose-dependent insulinotropic polypeptide, is synthesized naturally in the small intestine. This polypeptide binds to the GIP receptor to inhibit gastric acid secretion and gastrin release while stimulating insulin release. The latter is the primary function of GIP-R and is the primary reason that insulin levels increase following a meal.
Glucagon-like peptide-1 receptors are found on beta cells as well as in neurons in the brain. Like GIP-R, stimulation of GLP-1R stimulates the release of insulin. Natural agonists include glucagon and GLP1, but it has also been shown to bind nearly a dozen synthetic agonists including dulaglutide, lithium, and oxyntomodulin. Activation of GLP-1R increases both insulin synthesis and insulin release, factors that have made it a desirable target in drug development. In the brain, GLP-1R stimulation lowers appetite.
Interestingly, stimulation of GLP-1R appears to increase beta cell density in the pancreas. GLP-1R stimulation increases expression of the anti-apoptotic bcl-2 gene while reducing expression of pro-apoptotic bax and caspase-3 genes. This leads to enhanced beta cell survival and, ultimately, to increased levels of insulin[4].
The combination of GIPR and GLP-1R activity is what gives Tirzepatide an edge over strict GLP-1R agonists. Research shows that Tirzepatide acts identically to GIP at the GIPR, but favors cAMP production over β-arrestin recruitment when acting at the GLP-1R. These details may seem esoteric to some extent, but this difference in activity from endogenous GLP-1 appears to cause GLP-1R activation without increasing physiological internalization of the receptor. The net result is enhanced GLP-1R activity with Tarazepide compared to both endogenous GLP-1 as well as other synthetic GLP-1R agonists[5]. These slight alterations mean that Tirzepatide drastically enhances insulin secretion, promotes feelings of satiety, and reduces inflammation in adipose tissue. These combined effects make it a highly efficacious anti-diabetes peptide.
Finally, Tirzepatide appears to alter adiponectin levels, raising overall levels of the fat-burning peptide. Increased levels of adiponectin reduce fat cell differentiation and increase energy expenditure by making mitochondria more inefficient. A low level of this peptide hormone has been implicated in diseases such as type 2 diabetes, atherosclerosis, and non-alcoholic fatty liver disease[6]. It is worth noting that elevated adiponectin levels elevate insulin sensitivity, so it would appear that Tirzepatide modulates insulin sensitivity via several mechanisms.
Tirzepatide and Hunger
Research shows that Tirzepatide delays gastric emptying during the earliest phases of its administration but that the effect diminishes over time as a result of tachyphylaxis[7]. These effects are similar to those seen with pure GLP-1R agonists, indicating that this action of Tirzepatide is almost completely controlled by its GLP-1 activity and not at all by its GIP activity.
It appears that the effects of Tirzepatide on gastric emptying can be prolonged if the peptide is taken at a low dose for four weeks and then the dose is escalated. This also helps to mitigate side effects caused by the peptide and creates a veritable win-win for patients. Delayed gastric emptying can help to increase feelings of satiety and reduce hunger as well as food cravings. Combined with the effects Tirzepatide has on glucose levels, this can actually help to alter eating patterns over the long term.
Tirzepatide and Weight
As noted above, Tirzepatide use is associated with substantial weight loss over a six-month time interval. A comparison of Tirzepatide to other GLP-1 analogues, like degludoc, indicates a striking difference. Whereas Tirzepatide causes a dose-dependent decrease in weight over time, degludoc and other GLP-1R agonists cause weight gain[12].
It appears that the GIP agonism cause by Tirzepatide is what is responsible for the peptide’s long-term effects on weight. GIP appears to directly impact the insulin-sensitivity of adipocytes, which is likely the mechanism by which Tirzepatide impacts adiponectin levels. In short, Tirzepatide activates GIP receptors in fat cells, which then leads to an increase insulin sensitivity. This, in turn, leads to a reduction in adipose inflammation as well as an increase in adiponectin levels and the associated benefits. This isn’t the whole picture, however.
Research shows that GIP signaling in the central nervous system regulates hypothalamic feeding centers leading to decreased food intake and improved glucose handling. This, in turn, leads to decreased body weight[13]. Thus, it appears that Tirzepatide impacts weight via adiponectin signaling directly in adipose tissue and via CNS alterations that reduce hunger levels via GIPR signaling in the brain.
Tirzepatide and the Heart
As noted, Tirzepatide alters adiponectin levels. Low adiponectin has been associated with atherosclerosis, obesity, and heart disease while increased adiponectin levels have been associated with decrease risk of all of these things. Research in humans with type 2 diabetes has shown that Tirzepatide improves lipoprotein biomarkers, lowering levels of triglycerides, apoC-III, and a handful of other lipoproteins[8]. Combined, these effects mean reduced risk of heart disease as a likely result of decreased adiposity. Research shows that increased adiponectin levels increase HDL levels while decreasing triglyceride levels, both of which are associated with lower risk of heart disease. The peptide hormone appears to go further though, reducing scavenger receptors in macrophages and increasing the levels of cholesterol efflux to greatly protect against atherosclerosis. Increases in adiponectin levels have been associated with improved nutrition, exercise, and the use of certain lipid-lowering medications[9]. It appears that Tirzepatide has similar beneficial effects.
Research shows that GLP-1 is important in both the direct regulation of cardiovascular risk factors such as hypertension, dyslipidemia, and obesity as well as in the indirect regulation of risk factors like inflammation and endothelial cell dysfunction[10]. The former effects are discussed above and below in relationship to adiponectin. The effects on inflammation and endothelial function, however, appear to be mediated more directly.
In the case of endothelial function, GLP-1 signaling has been shown to induce relaxation of blood vessels leading to decreased blood pressure and enhanced end organ perfusion. This effect appears to result from increased expression of eNOS, the enzyme that generates nitric oxide and induces vascular relaxation. Interestingly, these effects appear to be enhanced in the setting of preexisting cardiovasulcar disease and diabetes[10].
Of course, it is well known that inflammation is directly correlated with atherosclerosis. The details are still being worked out, but GLP-1 signaling appears to decrease inflammation via a handful of mechanisms including reduced NF-κB signaling, decreased MMP-9 activity, inhibited inflammatory cytokine synthesis, and decreases in inflammatory macrophage activity. What is more, these effects appear to last as long as three months after a single dose of a GLP-1R agonist like Tirzepatide[10]. Tirzepatide is undergoing a clinical trial to further evaluate its medium-term effects on individuals with heart failure[11].
Tirzepatide Summary
Tirzepatide is a synthetic derivative of gastric inhibitory polypeptide (GIP) that has simultaneous glucagon-like peptide-1 (GLP-1) functionality as well. This combination allows Tirzepatide to lower blood glucose levels, increase insulin sensitivity, boost feelings of satiety, and accelerate weight loss. Tirzepatide was developed to fight type 2 diabetes, but has additionally been shown to protect the cardiovascular system and act as a potent weight loss agent.
In 1986 Professor Jens Juul Holst discovered the GLP-1 hormone in connection with his work on stomach ulcer surgery. Since the discovery, Novo Nordisk have used the research to successfully develop products to treat diabetes and obesity. The hormone GLP-1 can be used to regulate blood sugar levels and satiety. Not only has it made treatment of obesity and diabetes possible, it has also proven useful preventatively through early diagnosis for citizens who are at risk of developing diabetes and obesity. In 2015, Jens Juul Holst received the prestigious international Fernström prize for his research on GLP-1. He is one of the most cited researchers in Europe, with over 1,200 published articles and citations in over 3,500 articles annually.
- “The Physiology of Glucagon-like Peptide 1 | Physiological Reviews.” [Online].
- “Combined treatment with lisofylline and exendin-4 reverses autoimmune diabetes. - PubMed - NCBI.” [Online].
- “The proglucagon-derived peptide, glucagon-like peptide-2, is a neurotransmitter involved in the regulation of food intake. - PubMed - NCBI.” [Online].
- “Interim analysis of the effects of exenatide treatment on A1C, weight and cardiovascular risk factors over 82 weeks in 314 overweight patients with... - PubMed - NCBI.” [Online].
- “Cardiac function in mice lacking the glucagon-like peptide-1 receptor. - PubMed - NCBI.” [Online].
- “Glucagon-like Peptide 1 Can Directly Protect the Heart Against Ischemia/Reperfusion Injury | Diabetes.” [Online].
- “Recombinant glucagon-like peptide-1 increases myocardial glucose uptake and improves left ventricular performance in conscious dogs with pacing-ind... - PubMed - NCBI.” [Online].
- “Glucagon-like peptide-1 receptor is involved in learning and neuroprotection. - PubMed - NCBI.” [Online].
- “Protection and reversal of excitotoxic neuronal damage by glucagon-like peptide-1 and exendin-4. - PubMed - NCBI.” [Online].
- “A new Alzheimer’s disease interventive strategy: GLP-1. - PubMed - NCBI.” [Online].
- Holst JJ. From the Incretin Concept and the Discovery of GLP-1 to Today's Diabetes Therapy. Front Endocrinol (Lausanne). 2019;10:260. Published 2019 Apr 26. doi:10.3389/fendo.2019.00260 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6497767/